Abstract
Introduction: Ponatinib is a potent, oral, third-generation tyrosine kinase inhibitor (TKI) that is FDA approved for treatment of patients with relapsed/refractory CML. Patients with resistant or intolerant CP-CML demonstrated deep, lasting responses to ponatinib 45 mg once daily in the pivotal phase 2 PACE trial (Ponatinib Ph+ ALL and CML Evaluation, NCT01207440; completed). The phase 2 OPTIC trial (Optimizing Ponatinib Treatment in CP-CML, NCT02467270; ongoing) prospectively evaluated a response-based dose-reduction strategy in an attempt to optimize the dose schedule of ponatinib in patients with CP-CML resistant to second-generation (2G) BCR-ABL1 TKI therapy or with a T315I mutation. Unique dosing strategies in the 2 trials (PACE and OPTIC) provide the opportunity to closely evaluate the dose and schedule of ponatinib. Here, we conduct an in-depth analysis of dosing dynamics between the 2 trials and compare efficacy and safety outcomes.
Methods: In this analysis, adults with resistant or intolerant CP-CML from the PACE and OPTIC trials were enrolled and received an initial dose of ponatinib 45 mg once daily (PACE) or were randomly assigned (1:1:1) to an initial oral dose of ponatinib 45 mg, 30 mg, or 15 mg once daily (OPTIC). In PACE, proactive dose reductions were mandated in ≈2 years from initiation of first patient (in 2013) as arterial occlusive events (AOEs) emerged as notable adverse events (AEs); patients who achieved major cytogenetic response (MCyR) had doses reduced to 15 mg once daily and those without MCyR reduced to 30 mg once daily, unless benefit-risk analysis justified treatment with a higher dose. OPTIC was designed to incorporate a mandatory response-based dose-reduction strategy; patients in the 45-mg and 30-mg cohorts in OPTIC achieving ≤1% BCR-ABL1 IS reduced their dose to 15 mg once daily; doses also were reduced to manage AEs. Data from patients with CP-CML in PACE and from the 45-mg starting dose cohort in OPTIC are included in this analysis. Efficacy outcomes included ≤1% BCR-ABL1 IS, progression-free survival (PFS), and overall survival (OS). Safety and dosing data are also presented, including treatment-emergent adverse events (TEAEs) and treatment-emergent AOE (TE-AOE) rates.
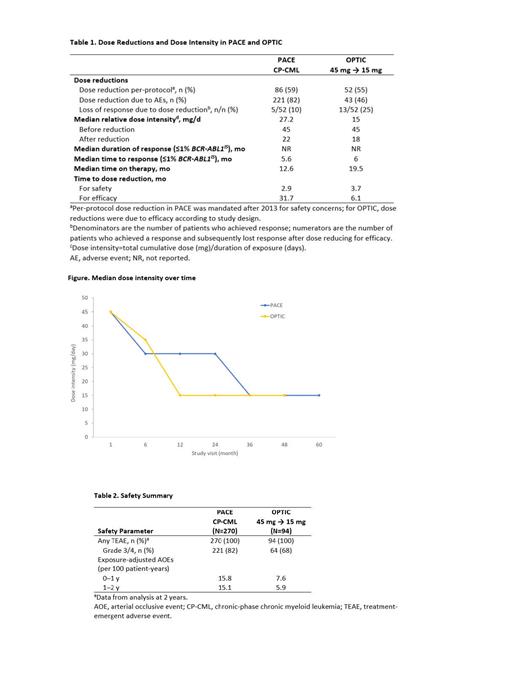
Results: Overall, 364 patients with CP-CML had at least 1 prior 2G TKI or had a T315I mutation and received a starting dose of ponatinib 45 mg (PACE, n=270; OPTIC, n=94). Median follow-up, 57 months (PACE) and 32 months (OPTIC). Efficacy outcomes were generally comparable or better in OPTIC when compared with PACE, including ≤1% BCR-ABL1 IS response by 24 months (PACE, 52%; OPTIC, 56%), 2-year PFS (68%; 80%), and 2-year OS (86%; 91%). Median time to ≤1% BCR-ABL1 IS response, 5.6 months (PACE) and 6 months (OPTIC). Median duration of response was not reached in either trial. Overall, median relative dose intensity (Table 1) was 27 mg/d in PACE and 15 mg/d in OPTIC, and dose reduction occurred more rapidly compared with PACE median (Figure). Dose reductions due to AEs occurred in 82% of patients in PACE and 46% in OPTIC. Median time to dose reduction for AEs was 2.85 months in PACE and 3.64 months in OPTIC. Median time on therapy was 12.6 months in PACE and 19.5 months in OPTIC. Exposure-adjusted TE-AOEs were 15.8 events per 100-patient years at 0 to <1 year in PACE and 7.6 events per 100-patient years at 0 to <1 year in OPTIC (Table 2). There were differences in baseline characteristics between the 2 trials, including differences in cardiovascular baseline status; however, these were accounted for in the propensity score analyses comparing AOE incidence, which after adjusting for baseline differences, showed a 60% reduction in relative risk for AOEs in OPTIC vs PACE. In-depth individual dosing dynamics by safety and efficacy will be presented.
Conclusions: The response-based dose-reduction strategy in the OPTIC trial resulted in more rapid dose reductions, lower overall median relative dose intensity, fewer dose reductions related to AEs, and longer median time on therapy in OPTIC compared with PACE, further demonstrating the benefit of the response-based dosing regimen used in OPTIC. These data from the PACE and OPTIC trials suggest that treatment with a response-based dose-reduction strategy may provide comparable or better efficacy while mitigating risk of AEs/AOEs with ponatinib. Furthermore, this abstract supports the rationale to explore response-based dose-modification strategies for other BCR-ABL1 TKIs.
Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding. Deininger: SPARC, DisperSol, Leukemia & Lymphoma Society: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Part of a Study Management Committee, Research Funding; Novartis: Consultancy, Research Funding; Fusion Pharma, Medscape, DisperSol: Consultancy; Sangamo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Part of a Study Management Committee, Research Funding; Incyte: Consultancy, Honoraria, Research Funding. Abruzzese: Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria. Apperley: Bristol Myers Squibb, Novartis: Honoraria, Speakers Bureau; Incyte, Pfizer: Honoraria, Research Funding, Speakers Bureau. Cortes: Bristol Myers Squibb, Daiichi Sankyo, Jazz Pharmaceuticals, Astellas, Novartis, Pfizer, Takeda, BioPath Holdings, Incyte: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Sun Pharma: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Bio-Path Holdings, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees. Chuah: Pfizer: Other: Travel, Research Funding; Steward Cross: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Novartis, Korea Otsuka Pharmaceutical: Honoraria. DeAngelo: Abbvie: Research Funding; Takeda: Consultancy; Servier: Consultancy; Pfizer: Consultancy; Novartis: Consultancy, Research Funding; Jazz: Consultancy; Incyte: Consultancy; Forty-Seven: Consultancy; Autolus: Consultancy; Amgen: Consultancy; Agios: Consultancy; Blueprint: Research Funding; Glycomimetrics: Research Funding. Hochhaus: Bristol-Myers Squibb: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Incyte: Research Funding. Lipton: Bristol Myers Squibb, Ariad, Pfizer, Novartis: Consultancy, Research Funding. Nicolini: BMS: Honoraria; Sun Pharma Ltd.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte Biosciences: Honoraria, Other: travel, accommodations, expenses, Research Funding, Speakers Bureau; Kartos Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel, accommodations, expenses, Research Funding. Pinilla Ibarz: AbbVie, Janssen, AstraZeneca, Takeda: Speakers Bureau; MEI, Sunesis: Research Funding; Sellas: Other: ), patents/royalties/other intellectual property; AbbVie, Janssen, AstraZeneca, Novartis, TG Therapeutics, Takeda: Consultancy, Other: Advisory. Rea: Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees. Rosti: Pfizer: Research Funding, Speakers Bureau; Bristol Myers Squibb, Incyte, Novartis: Speakers Bureau. Rousselot: Incyte, Pfizer: Consultancy, Research Funding. Mauro: Novartis: Consultancy, Research Funding; Takeda: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Pfizer: Consultancy; Sun Pharma / SPARC: Research Funding. Shah: Bristol-Myers Squibb: Research Funding. Talpaz: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Other: Grant/research support ; Constellation: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Imago: Consultancy; Celgene: Consultancy. Vorog: Takeda: Current Employment. Lu: Takeda: Current Employment. Kantarjian: Daiichi-Sankyo: Research Funding; AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; BMS: Research Funding; Astra Zeneca: Honoraria; Ipsen Pharmaceuticals: Honoraria; KAHR Medical Ltd: Honoraria; Pfizer: Honoraria, Research Funding; Jazz: Research Funding; Novartis: Honoraria, Research Funding; Astellas Health: Honoraria; NOVA Research: Honoraria; Ascentage: Research Funding; Aptitude Health: Honoraria; Immunogen: Research Funding; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal